On January 30, the Food and Drug Administration announced it approved the first of a new class of pain reliever drugs as an alternative to opioids. Journavx (generic name suzetrigine) was found to work as well as hydrocodone (Vicodin, Norco) to treat moderate to severe postoperative pain in two Phase 3 clinical trials, one with patients who underwent bunionectomy surgery and another on patients who underwent abdominoplasty.
The drug is unique in that, unlike opioids, which directly target the brain to dull the perception of pain, or non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen and naproxen, which reduce inflammation and pain at the injury site, suzetrigine blocks the nerves that transmit pain signals to the brain.
The Phase 1 trials of suzetrigine took place in 2021. The entire approval process took only about four years, which is not typical for the FDA. The process usually takes about 10–15 years, and the Phase 1–3 clinical trials process generally takes around 6–7 years, followed by 10 months for standard FDA review (6 months for accelerated review). According to a report from the trade association BIO, 90 percent of drugs don’t make it through the Phase 1 approval process. A 2024 study published in the Journal of the American Medical Association estimated the mean cost of bringing a drug to market from 2000 to 2018 to be “$879.3 million when both drug development failure and capital costs were included.”
The FDA accelerated suzetrigine’s approval through its Overdose Prevention Framework, streamlining the clinical trial and approval process for opioid substitutes and harm-reduction products such as overdose antidotes. This demonstrates that the FDA can dramatically reduce the regulatory burden on new drug development when it deems a problem urgent. However, the FDA—not the patients suffering from specific conditions—decides what qualifies as urgent.
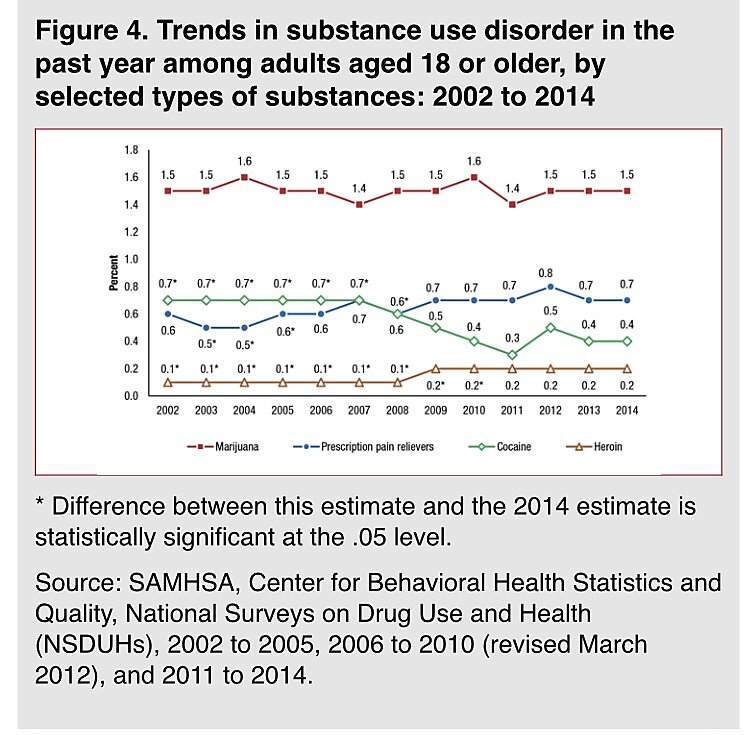
The FDA created the accelerated framework based on the debunked narrative that the overdose crisis was caused by doctors hooking their patients on opioids. As I have written, the overdose crisis resulted from a growing population of nonmedical drug users accessing drugs in the increasingly dangerous black market caused by drug prohibition. Substance Abuse and Mental Health Services Administration (SAMHSA) surveys begun in 2002 consistently show the addiction rate to prescription pain pills has never exceeded 0.8 percent of adults over age 18 (see attached graph).
As early as 2017, I pointed out that overdose rates began to climb as doctors started cutting back on opioid prescribing, which reduced nonmedical users’ access to diverted pain pills and drove them to more dangerous heroin and, now, fentanyl.
Nevertheless, Journavx appears to be a real breakthrough drug, opening the door for a new class of pain relievers for patients to access. This is good news.
A caveat: Pain is subjective, and analgesics affect individuals differently. The clinical trials found it roughly equivalent to a standard dose of hydrocodone. Hydrocodone is not as potent as oxycodone and doesn’t work for everyone, though it works for many people.
Clinicians must still be ready to prescribe oxycodone or something even more potent if this new drug fails to provide the relief patients require. I worry that many doctors might resort to prescribing this drug exclusively and abandon opioid alternatives altogether due to fear of disciplinary action from cops practicing medicine.